
This value is called Avogadro’s number, or Avogadro’s constant. One mp of substance is defined as equivalent to the number of carbon atoms present in 12 grams of the isotope carbon 12, which is about 6,022 x 10 23 atoms. To be able to measure an exact amount of a substance, scientists use a unit called mp to represent a definite number of atoms.

The size of an atom is so small that it is difficult to accurately measure the number of atoms of a chemical compound. One way to remember atoms with two molecules is to memorize the sentence: “Have No Fear Of Ice Cpd Beverages” (the initials are for Hydrogen, Nitrogen, Fluorine, Oxygen, Iodine, Chlorine, Bromine).That is, if you want to calculate the mass mp of compounds made up of more than one atom, such as hydrogen gas, oxygen gas or chlorine gas, you need to determine the average atomic mass of the compound and then multiply this value with the mass constant mp, ”then” multiplies the product just found by 2. Some elements exist in nature as molecules consisting of two or more identical atoms.0107 grams per mp, for oxygen 15.9995 grams per mp, and for chlorine 35,453 grams per mp. The product of average atomic mass and mass constant mp will convert units of atomic mass to grams per mp, so the mp mass of hydrogen will be 1,007 grams per mp, of carbon 12. The unit of mass mp is defined as 0.001 kilogram per mp, or 1 gram per mp.
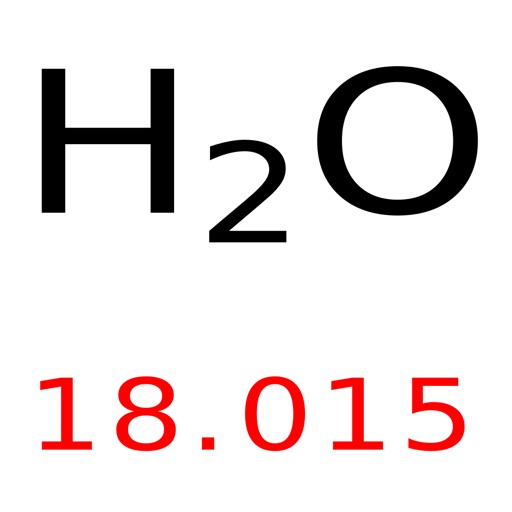
Multiply the average atomic mass by the mass constant mp.


 0 kommentar(er)
0 kommentar(er)
